
Pharmacovigilance & Individual Case Safety Reports
Inconsistency in the adverse event reporting process is a common problem that may have a serious economic impact for pharmaceutical companies.
The rise in the number of safety cases in recent years and the growth in the volume of data to be processed, has revamped pharmacovigilance processes.

What do we solve?
Currently, Individual Case Safety Report management is characterized by a poor reporting process where relevant information is omitted, the process of following instructions fails, or false-positives are being reported.
How do we solve it?
A smart data capture and analysis system is essential to streamline the adverse event reporting process by simplifying data capture, reducing errors, and improving the quality and standardization of reports. It enables scalability in volume, speed of processing and quality of capture.
1
Secure access
Access to the platform with personalized credentials via web (cloud)

1

Secure access
Access to the platform with personalized credentials via web (cloud)
Case Management
Create and modify adverse event reports supported by personalized alerts and ICH guidelines
2

Adverse event reporting
Using MNER-dezzai ontology
3

3

Adverse event reporting
Using MNER-dezzai ontology
Individual case follow-up
By status and priority
4

5
Documentation of the case process
And/or grouping by segmentation (Log + Findings)
5

Documentation of the case process
And/or grouping by segmentation (Log + Findings)
WHAT do we deliver?
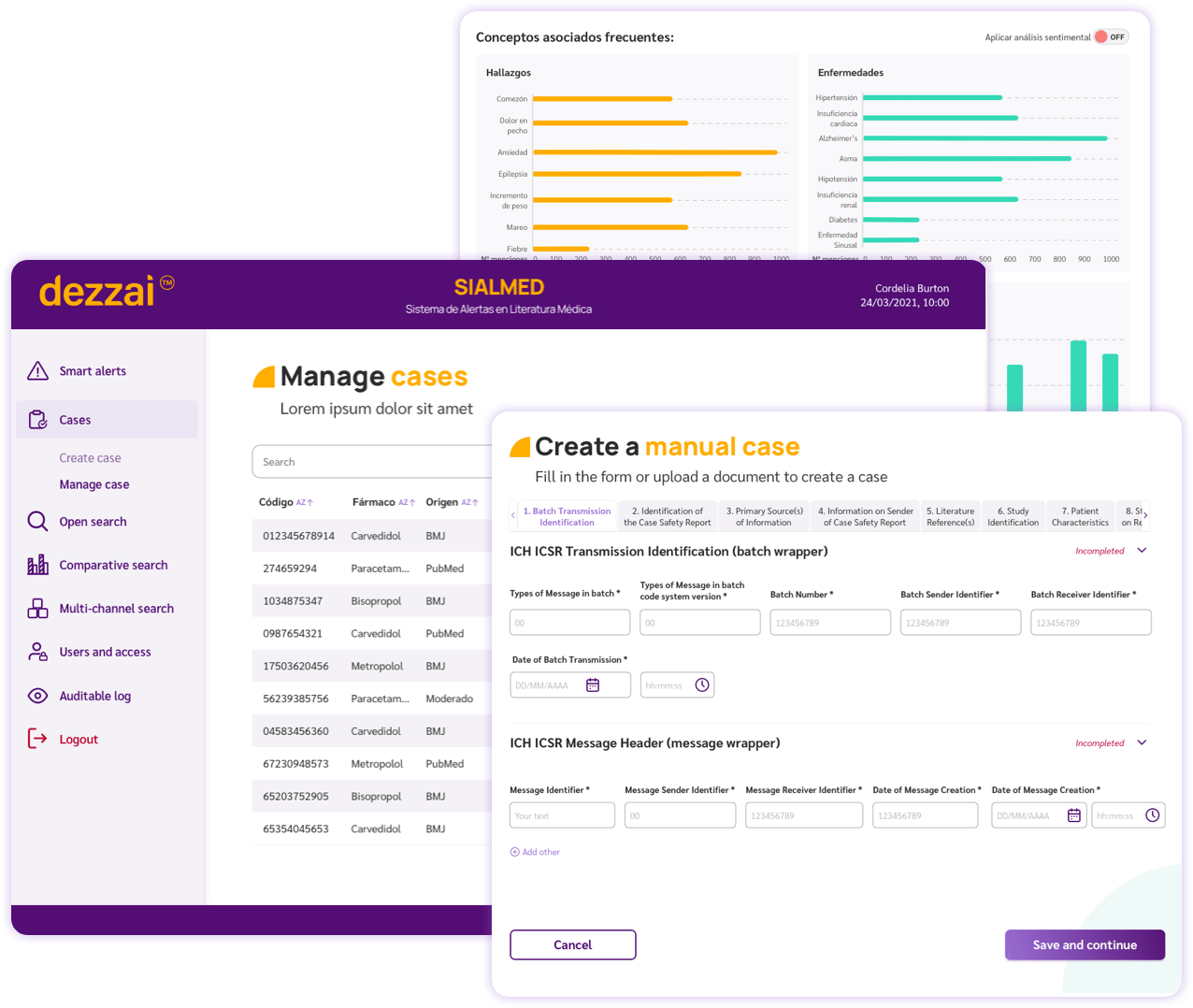
- ✅ Secure platform
Access with unique credentials
Permission management
Auditable reporting - ✅ Electronic data capture
Creation and management of adverse reports according to ICH regulations
Filling alerts by A.I.
Report coding with MedDRA, ICD-1 terminology - ✅ Report management
Segmentation and tracking of cases by priority and status
Exportable XML reports according to country regulations
Download our brochure
Download our brochure to have more information on the use case:
Other use cases
AI based Pharmacovigilance – Medical Literature
Smart Medical Writing
Real world evidence
Medical image

Medical text analytics
We’re here for you
We are certified by:






