
AI-based Pharmacovigilance: PMS – Medical Literature
Pharmacovigilance is a critical process in the pharma industry that can have a pivotal role in patient health outcomes and industry revenues.
Early detection and risk mitigation of adverse events is critical to avoid reputational crises, drug recalls and human losses that could severely impact a company’s revenue and profits.

What do we solve?
The traditional approach to pharmacovigilance relies on human resources to process and analyze multiple sources of information; but it is very costly and limited in terms of volume, speed, and depth.
How do we solve it?
Semantic A.I. can boost the capacity, speed, and depth of data processing and analysis using a combination of Natural Language Processing, Natural Language Generation, and Machine Learning technologies.
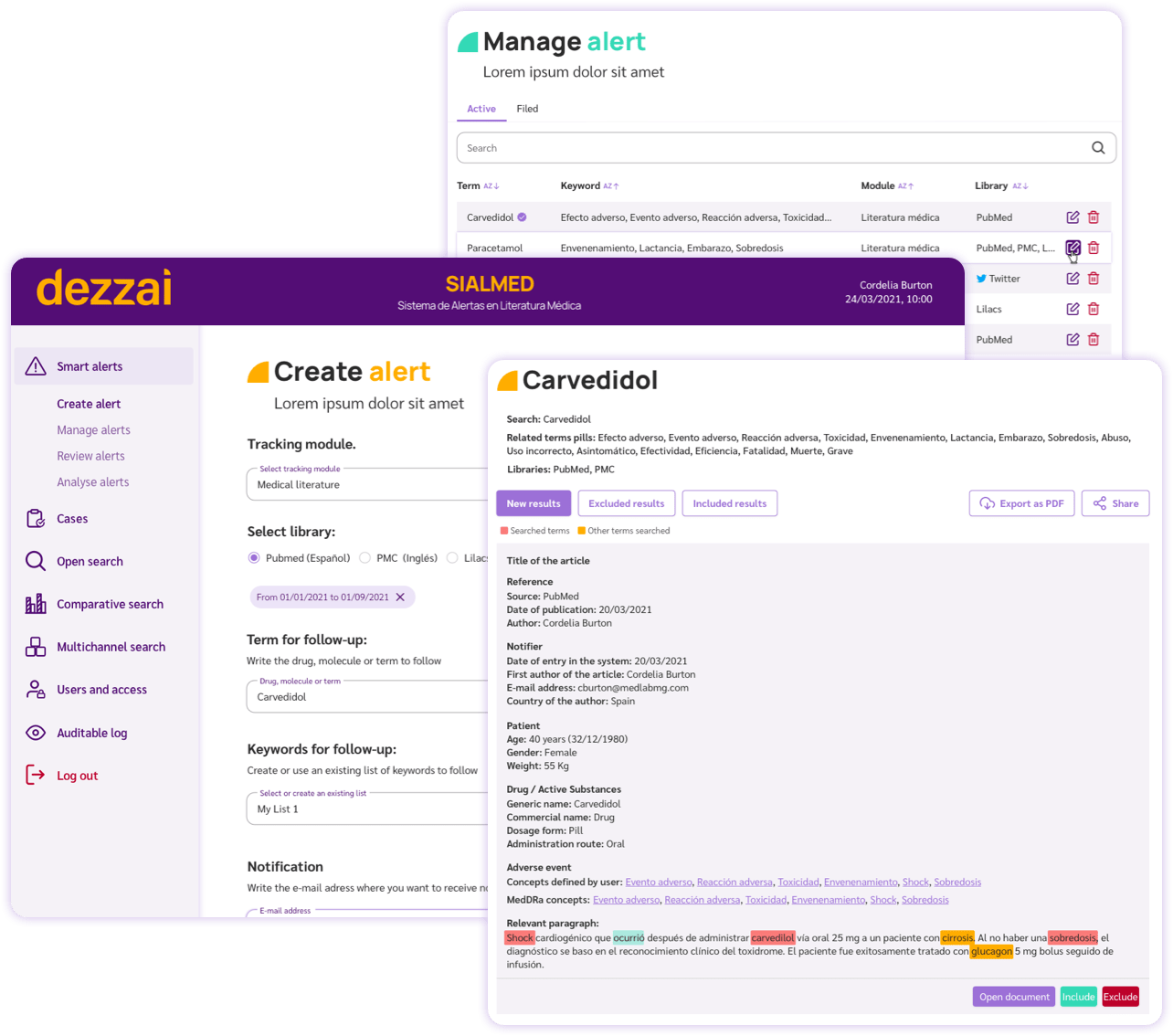
Search, extraction and consolidation
– Multi-language searches in various sources of medical literature with daily, weekly, monthly frequencies.
– Extraction and integration of documents containing potential adverse events according to user-defined parameters or MeDRA dictionary.
– Consolidation of a list of documents for review by a pharmacovigilance expert with A.I. support.
Detection, classification and validation
– Detection of adverse events according to the minimum criteria (Patient, Report, Event, Product) and other criteria for consideration.
Notice of criteria absence
Notice of quality failure
Safety event notification
– Assessment of seriousness or severity according to international or local definition.
– Evaluation of the casualty of the case according to algorithms (Naranjo, Kahrl and Lasagna, WHO, FDA, egtc).
Tracking, documentation and registration
– Case record including date of identification, key concepts, assets, source, reason for discard, security information.
– Case traking by creating alerts for missing requirements to supplement a case. Database consistency review to find duplicate, pending and incomplete cases.
– Export cases in PDF or XML format according to regulatory requirements.
What do we deliver?
dezzai provides the client with a modular platform to increase the capability of detection and analysis of drug related adverse events:
Smart connector
Automated extraction, and integration of adverse event reports from multiple channels (email, medical reports, call center, patient support programs, etc.)

Smart validator
Classification and validation of adverse events or quality related incidents using a human trained NLP model and standard information requirements.
Smart validator
Classification and validation of adverse events or quality related incidents using a human trained NLP model and standard information requirements.


Smart alerts
Automated rule-based notification system for adverse events, signals, incomplete requirements, or findings.
Smart reports
Reports or summarizations of findings to support the creation of compliance reports.


Smart reports
Reports or summarizations of findings to support the creation of compliance reports.
Easy-to-use, full compliance and flexible software
- ✅ Streamline search for possible adverse events
- ✅ Facilitate the classification and assessment of adverse events through A.I. algorithms in collaboration with a Pharmacovigilance expert.
- ✅ Simplify the monitoring, recording and documentation of adverse events
Download our brochure
Download our brochure to have more information on the use case:
Other use cases
AI based Pharmacovigilance – ICSR management
Smart Medical Writing
Real world evidence
Medical image

Medical text analytics
We’re here for you
We are certified by:






