
Artificial intelligence-powered platform for adverse event case management and post-marketing surveillance.
Adverse event review with an intuitive, customisable, automated and industry compliant software.

What do we solve?
Early detection and risk mitigation of adverse events is critical to avoid reputational crisis, drug recalls and human losses that could severely impact a company’s revenue and profit. The traditional approach to pharmacovigilance relies on human resources to process and analyse adverse events from multiple sources of information.
It is a very costly and limited process in terms of volume, speed and quality of analysis.
How do we solve it?
dezzai Sentria uses semantic AI technology to enhance the capacity, speed and precision of adverse event management by following the next steps:

Search, extraction and pre-screening of cases of adverse events.

Detection, classification, and validation of potential ICSR pharmacovigilance events

Tracking, documenting and registration of case of adverse events.
What do we deliver?
dezzai provides the client with a modular pharmacovigilance platform to increase the capability of detection and analysis of drug monitoring and adverse events:
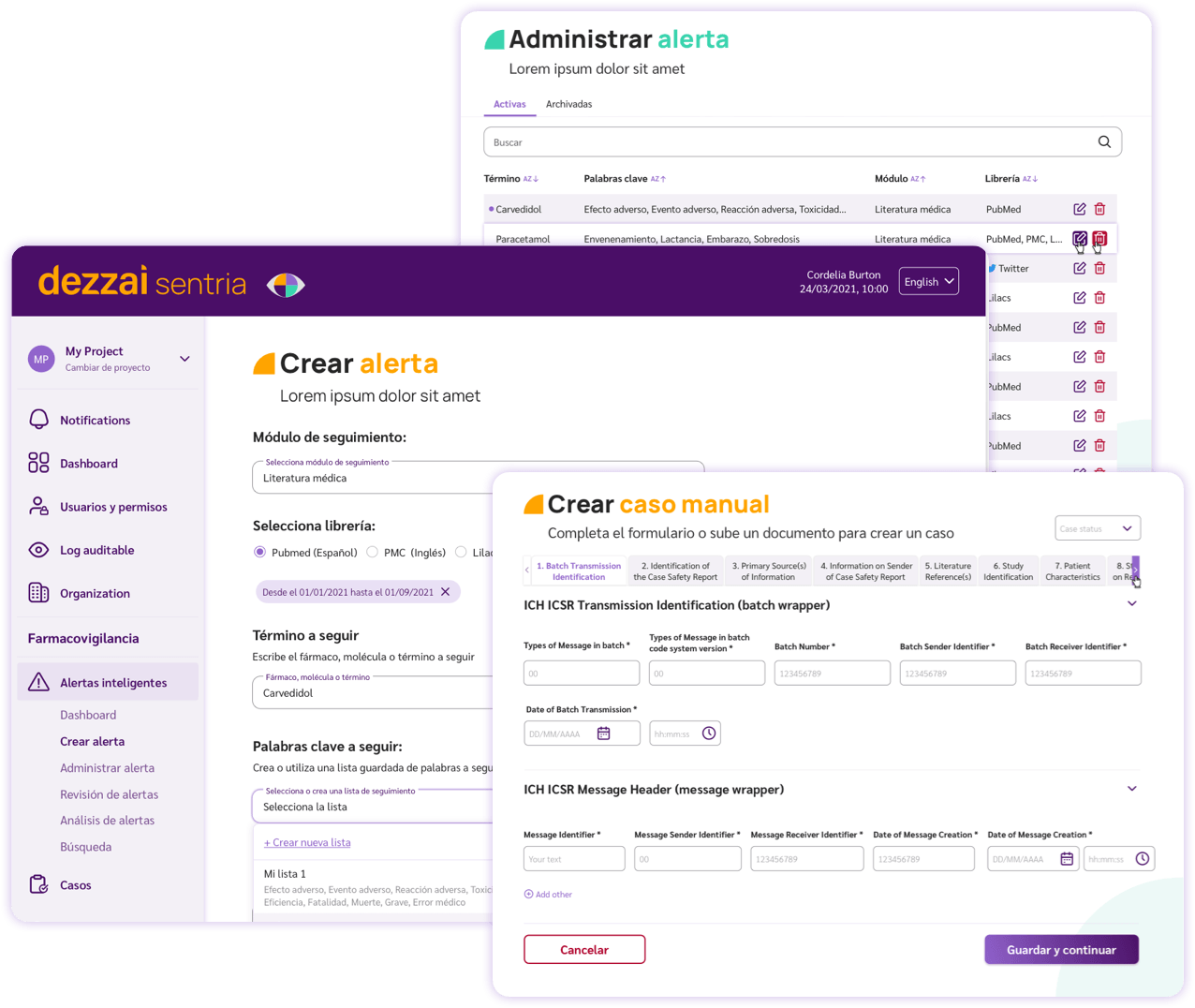
- ✅ Facilitate pharmacovigilance compliance with ICH international regulatory guidelines (E2B R3).
- ✅ Ensure the proper evaluation of adverse events and the correct execution of work procedures following local regulations.
- ✅ Streamline capturing reports through an intuitive interface enhanced by pharmacovigilance automation.
- ✅ Reduce capture errors through intelligent alerts and facilitate report processing using international MedDRA, ATC/WHO coding.
- ✅ Increase analytical capabilities using semantic artificial intelligence for pharmacovigilance.
dezzai SENTRIA semantic AI technology to enhance the speed, volume, and precision of adverse event detection, classification, analysis, and documentation, integrating medical literature monitoring and digital pharmacovigilance methods to provide a comprehensive pharmacovigilance solution. This approach enables effective pharmacovigilance management and ensures that the latest advancements in the field are incorporated into our system, offering a cutting-edge, efficient, and reliable pharmacovigilance system for our clients.

Secure platform
– Access controlled with credentials
– Auditable log for compliance
– Encrypted cloud environment

Advanced search
– Languages: English / Spanish
– Sources: PubMed / Lilacs (possibility of inclusion of IBECS)
– Custom search algorithms
– Search filtering by period – dates
– Customizable search frequences and parameters

Alerts management
– New results notifications
– Custom search algorithms
– Assignable alerts to specific users

ICSR management
– Case creation: (ICH E2B R3)
• Electronic form
• Smart Alert
• Digital document
– Case management by priority and status
– Exportable XML reports according to country regulations
Algorith training module
– Classification by severity
– ICSR concept detection
– Exclusion by quality of other parameters
Download our brochure
Download our brochure to have more information on the use case:
Other use cases
AI based Pharmacovigilance – ICSR management
Smart Medical Writing
Real world evidence
Medical image

Medical text analytics
We’re here for you
We are certified by:






